Sialic Acid/Glycobiology:
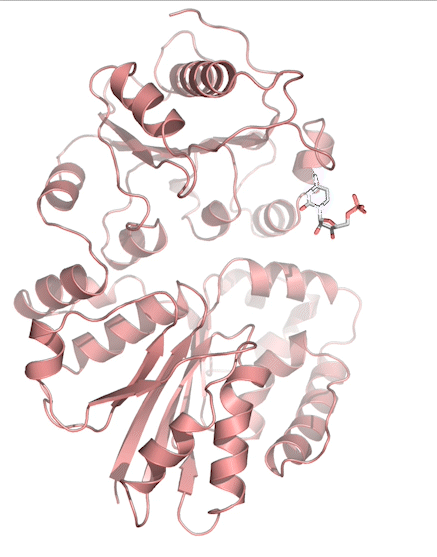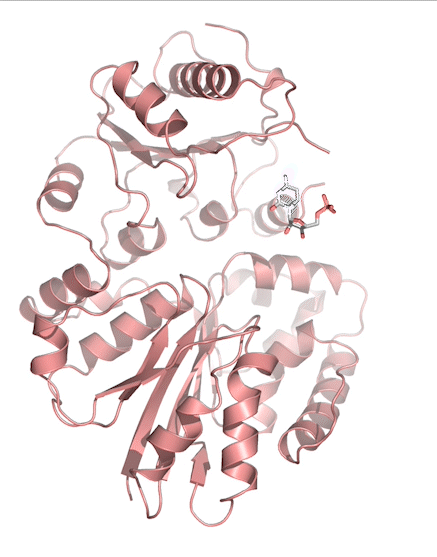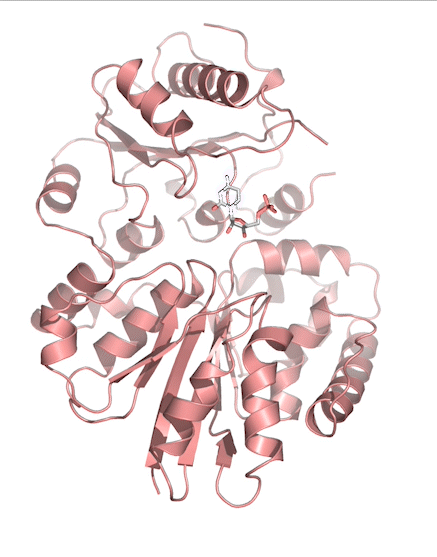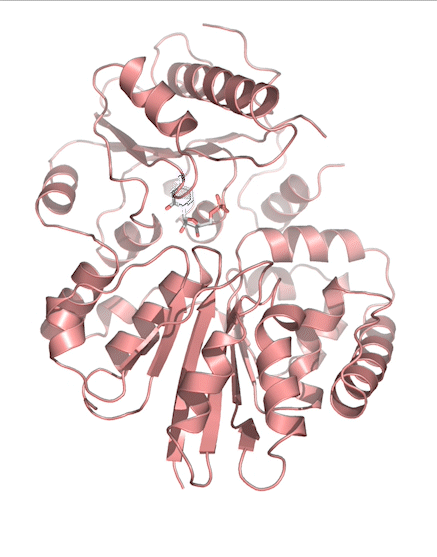
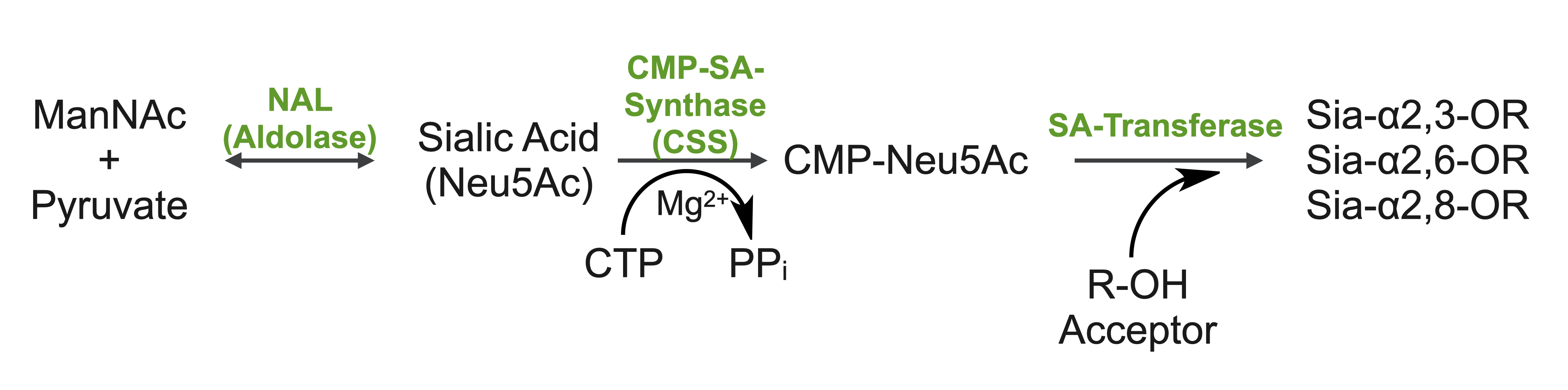
Sialic acids (N-acetylneuraminic acid) are nine-carbon acidic monosaccharides that are typically found at the terminus of cell-surface glycans. As the terminal carbohydrate residue, sialic acid is one of the first molecules encountered in cellular interactions and has been found to play important roles in cellular recognition, communication and pathogen attachment, such as influenza virus. Sialic acid has also been found on the surface of many pathogenic bacteria where they may function as molecular mimicry to evade the host’s immune system.
Our lab, in collaboration with the Chen lab, have been investigating the structure-function relationships of enzymes involved in the synthesis and conjugation of sialic acid. Our lab recently determined the crystal structures of the enzymes N-Acetylneuraminate lyase (NAL), also known as aldolase, CMP-sialic acid synthetase (CSS), and sialyltransferases, all with various substrate, product or substrate analogues. Despite their important biological functions, little is known about the molecular architecture and mechanism of these enzymes. Our goal of this project is to elucidate the molecular structure and catalytic mechanism of these enzymes with a long-term goal to find modulators to control diseases associated with both host and pathogen enzymes. Another aim is to use structure-guided protein engineering to modify the enzymes, which can be used in the chemoenzymatic synthesis of unnatural or modified sialic acid analogues for many diverse biotechnology applications.
